Andronotherapy - Ion therapy - Particle therapy
A leading consortium including CERN, GSI, Academia, leading industries, the four European ion therapy centres CNAO, HIT, MedAustron and UKGM, as well as SEEIIST - International Institute for Sustainable Technologies of South East Europe and funded by the EU.
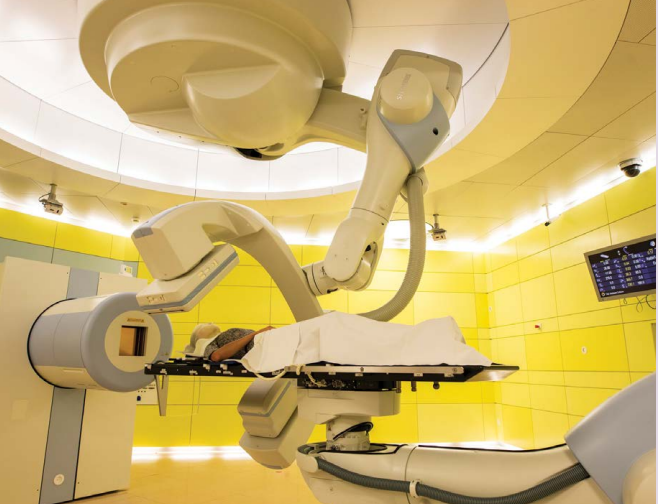
The project
Andronotherapy - Ion therapy - Particle therapy
The EU-funded HITRIplus (Heavy Ion Therapy Research Integration plus) project is an initiative to build a strong European research community on heavy ion therapy of cancer tumours. The project consortium includes all relevant stakeholders such as CERN, GSI, academia, leading EU industries, the four European ion therapy centres CNAO, HIT, MedAustron and UKGM, as well as SEEIST - the International Institute for Sustainable Technologies of South East Europe.
The aim of the HITRIplus project is to integrate and advance biophysical and medical research into the treatment of cancer with heavy ion beams.
The objective
Heavy ions in the battle to treat tumours
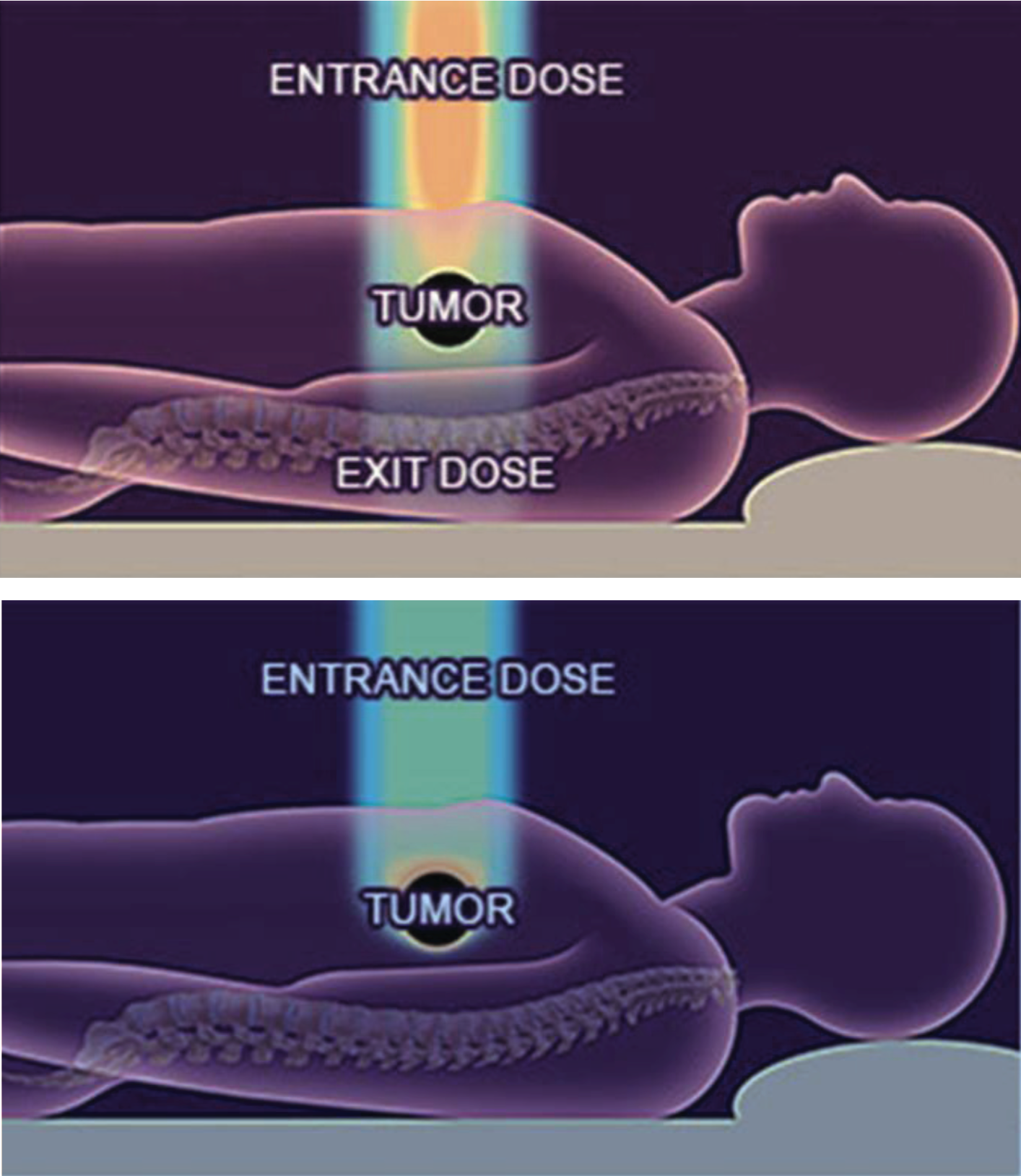
Heavy ion beam radiotherapy treats tumours by focusing with maximum precision on the diseased tissue while avoiding the healthy tissue around it. It is therefore more effective than any other form of conventional photon radiotherapy.
The broader goal of HITRIplus is to provide radiation oncologists with a cutting-edge tool for treating the fraction of tumours that are not treated as effectively with X-rays or protons and where treating these tumours with ions translates into better survival rates and lower recurrence rates.
The way
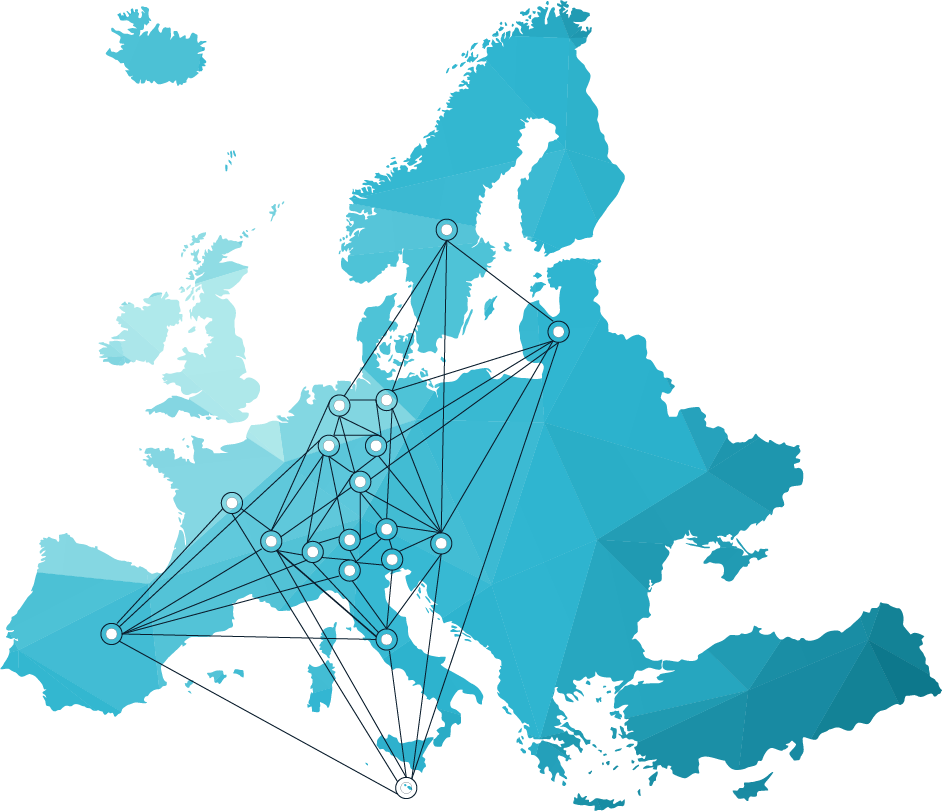
Partnership of the scientific community
The HITRIplus Transnational Access (TNA) brings together the heavy ion therapy centres operating in Europe and brings them together in a coordinated way to the medical and research community. Its network will structure and promote research on heavy ion therapy, including clinical and pre-clinical research. More specifically, the work programme aims to:
A) Clinical access, giving hospitals and oncology centres in Europe the opportunity to refer patients for treatment at existing andronotherapy facilities as well as to collaborate in clinical prospective research.
B) To link radiotherapy oncologists with their European and non-European colleagues in order to collaborate in multicentre prospective comparative studies to improve their knowledge of both heavy ion therapy and the improvement of classical radiotherapy oncology through clinical research practice.
The PAPAGEORGIOU Hospital of Thessaloniki
The Papageorgiou General Hospital, is the contact point for clinical access to the European Centres for Androtherapy.

Transnational - Clinical access to doctors and patients
In order to schedule a patient's visit to one of the androtherapy centres, the following must first be completed electronically using the form available at https://www.hitriplus.eu/transnational-access-ca/
Through the electronic form, the applicant will be able to the doctor (medical oncologist or radiotherapist) submit the patient's application for access to the TNA clinic available from HITRIplus partners.
Please note that in order for your application to be assessed, you must meet the following requirements:
- Have the patient consent to provide his or her details for the eligibility assessment committee.
- Send the necessary documentation in English, original or appropriately translated by clinical experts.
- Know and inform the patient that his/her data will be used for the sole purpose of assessing his/her case and your request for its treatment, and if the case is not positively assessed, it will be deleted within 3 months of your request.
The programme grants the amount of 3.000,00 €, which covers the expenses (travel, accommodation, etc.) of the doctor who wishes to accompany the patient to the treatment centre.
Persons insured under the Greek social security system have the right to travel abroad to access healthcare, also known as planned care, especially in cases where the care is not provided in Greece or cannot be provided within the medically acceptable timeframe for their health condition.
For its insured persons, EOPYY authorises cross-border healthcare (healthcare provided outside the patient's Member State of insurance) and pays all or part of the costs of the healthcare, upon submission of a request with a medical file/documents as defined in Articles 32 and 33 in Εthe National Health Care Benefits Regulation (EHIC).
Useful advice on organising planned treatment abroad and covering the cost of treatment/treatment can be found at https://eu-healthcare.eopyy.gov.gr
For further information on how to apply for access to the clinical access centres through the HITRIplus programme, please contact:
At Radiotherapy Department of the General Hospital of Thessaloniki "PAPAGEORGIOU
Ms. Maria Topalidou, Director of Radiation Oncology
email : rtherapypapageorgiouhospital@gmail.com ,tel. 2313 323420
Ms. Chione Kodonas, Radiophysicist,
email : medphys@papageorgiou-hospital.gr , tel. 2313 323419




